High-Pressure δ-Al(OH)3 and δ-AlOOH Phases and Isostructural Hydroxides/Oxyhydroxides: New Structural Insights from High-Resolution 1H and 27Al NMR | The Journal of Physical Chemistry B
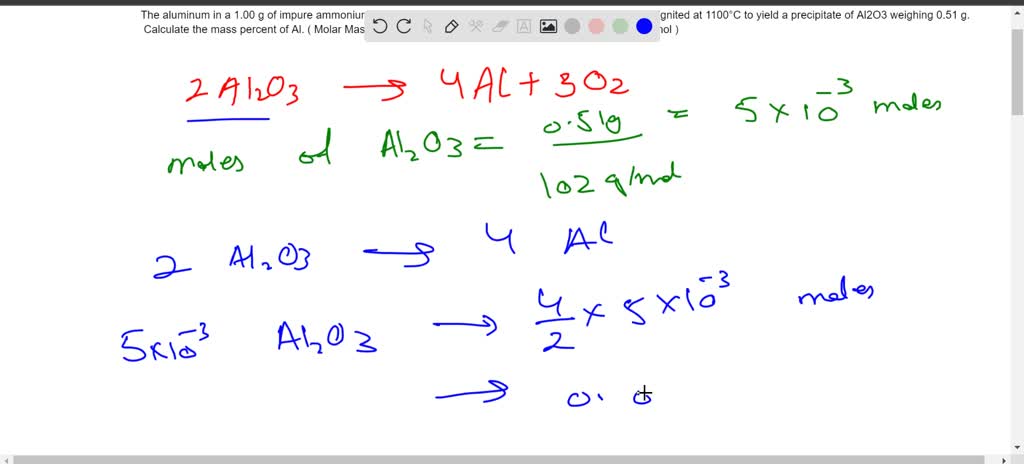
SOLVED: The aluminum in a 1.00 g of impure ammonium aluminum sulfate sample was precipitated as Al(OH)3 and ignited at 1100°C to yield a precipitate of Al2O3 weighing 0.51 g. Calculate the

Addition of Al(OH)3 versus AlO(OH) nanoparticles on the optical, thermo-mechanical and heat/oxygen transmission properties of microfibrillated cellulose films | SpringerLink

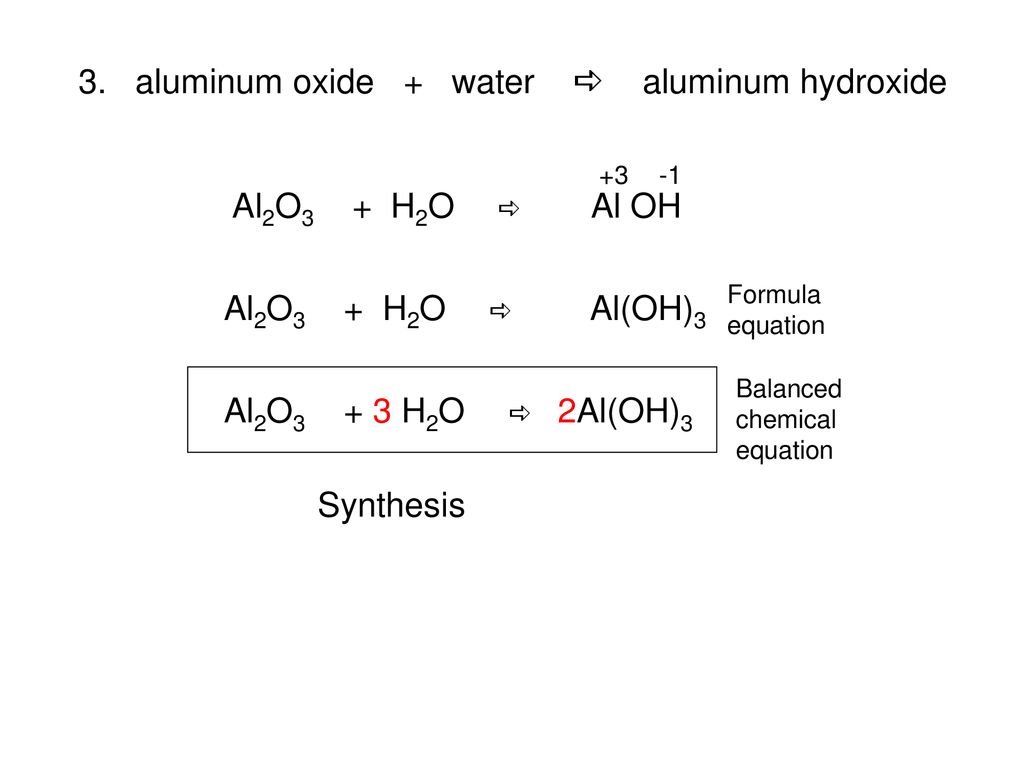
4/27/16 Today I will define stoichiometry and calculate mole-mole stoichiometry problems Warm Up Write a balanced equation for the reaction of magnesium. - ppt download

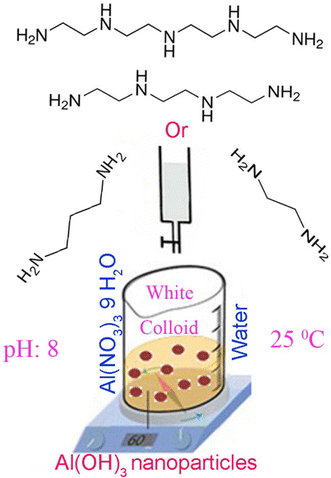
Synthesis of Al(OH) Nanostructures from Al(OH) Microagglomerates via Dissolution-Precipitation Route

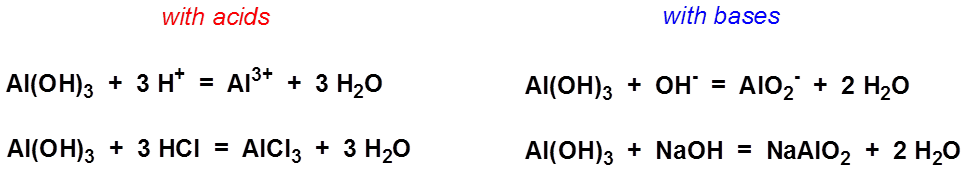
Al-alcl3-al(oh)3-al2o3-и посложнее )) (На картинке ) Составьте уравнения :) Спасибо заранее - Школьные Знания.com